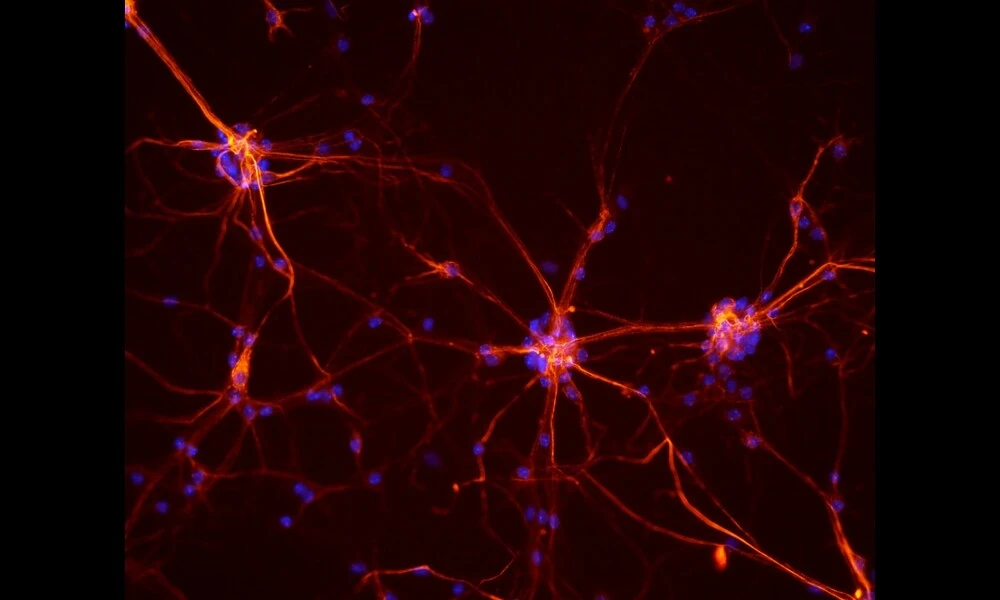
Mitochondrial Protein MCU Important For Neuronal Synaptic Plasticity and Memory Formation
Published on Sat Nov 11 2023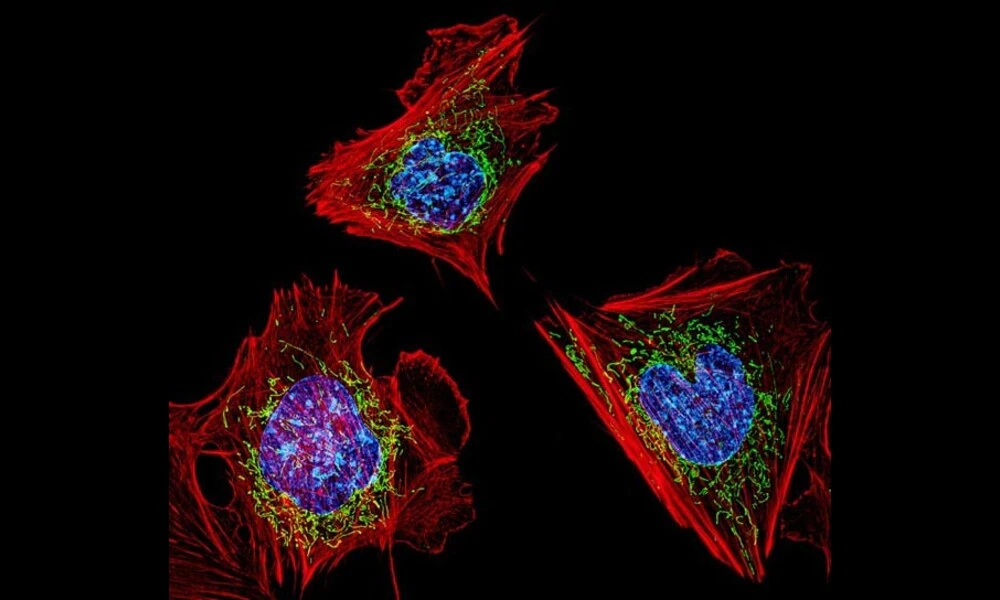 Cells with nuclei in blue, energy factories in green and the actin cytoskeleton in red | NIH Image Gallery on Flickr
Cells with nuclei in blue, energy factories in green and the actin cytoskeleton in red | NIH Image Gallery on FlickrMitochondria, the powerhouse of cells, have long been known for their crucial role in energy production. However, recent research is shedding light on their involvement in other important cellular functions, such as synaptic plasticity in neurons. A preprint paper titled "The mitochondrial calcium uniporter is necessary for synaptic plasticity and proper mitochondrial morphology and distribution in the distal dendrites of CA2 neurons" delves into the mechanisms governing synaptic plasticity and the role of a specific mitochondrial protein in this process.
The preprint paper focuses on the mitochondrial calcium uniporter (MCU), a channel responsible for regulating calcium levels within mitochondria. Calcium influx through this channel has been shown to impact mitochondrial function and distribution, thereby influencing cellular energy production. The researchers investigated the role of MCU in synaptic plasticity within the dendrites of CA2 neurons, a specific region of the hippocampus associated with social memory.
Using genetically modified mice lacking MCU specifically in CA2 neurons, the researchers discovered that the absence of MCU led to a deficit in long-term potentiation (LTP) at synapses located in the distal dendrites of CA2 neurons. LTP is a process crucial for memory formation and is known to be impaired in certain neuropsychiatric disorders. Additionally, the loss of MCU correlated with decreased spine density in these distal dendrites, indicating an alteration in the structural plasticity of the synapses.
Furthermore, the researchers observed that the absence of MCU caused significant changes in the morphology of mitochondria in the CA2 dendrites. Mitochondria were found to be smaller and more fragmented in the absence of MCU compared to control mice. These alterations in mitochondrial structure may lead to functional changes, such as decreased ATP production, which could underlie the deficits in synaptic plasticity observed in the study.
These findings emphasize the critical role of mitochondrial calcium regulation in synaptic plasticity and memory formation. The specific enrichment of MCU in the distal dendrites of CA2 neurons suggests that this protein plays a unique role in promoting LTP in this region compared to neighboring CA1 and CA3 neurons. Understanding the mechanisms underlying synaptic plasticity is crucial for unraveling the complexities of memory formation and cognitive processes, as well as for developing potential therapies for conditions associated with memory deficits.
In conclusion, this preprint paper highlights the importance of the mitochondrial calcium uniporter in the regulation of synaptic plasticity and proper mitochondrial function. The findings pave the way for future research into the diverse roles of mitochondria in cellular function and their potential implications in neurological disorders that involve impaired synaptic plasticity.